How Regulatory Writing Empowers You to Help More Patients
Can Writing Help More Patients Than Clinical Practice?
Hey there, Burned-Out Clinicians!
Feeling like patient caps, packed schedules, and endless bureaucracy limit your impact?
I’ve been there, questioning if all my years of training could ever translate to something bigger. I was seeing patients, but I knew the system was holding me back from making the kind of difference I dreamed of. You can only do so much when your impact is measured one appointment at a time.
But what if your skills could help millions instead of dozens?
That’s the opportunity regulatory medical writing gives you.
Today, I’ll discuss how this field allows you to escape the grind of clinical practice while making an even greater impact on patient lives.
We’ll explore:
what regulatory writing is
how your clinical expertise prepares you for it
the non-writing job most newcomers use to get a foot in the door.
By the end, you’ll see how this career path lets you help more patients than ever while enjoying the flexibility and growth you deserve.
Let’s get started!
What is Regulatory Writing?
Regulatory medical writing produces the critical documents regulatory agencies (FDA, EMA, etc.) rely on to evaluate and approve new medical products.
If you’ve ever wondered how your clinical skills could translate for broader impact, consider the following roles regulatory writing plays:
Helping companies meet stringent requirements for public health
Communicating complex clinical data in a clear, structured way
Supporting the development of life-changing treatments
Many healthcare professionals find themselves stuck in roles that don’t allow them to grow despite their expertise. Regulatory medical writing provides a rewarding opportunity to apply your skills while offering greater flexibility, intellectual challenges, and a chance to influence global health.
This career path transforms your existing knowledge into meaningful contributions, allowing you to help more patients while enjoying a balanced and fulfilling professional life.
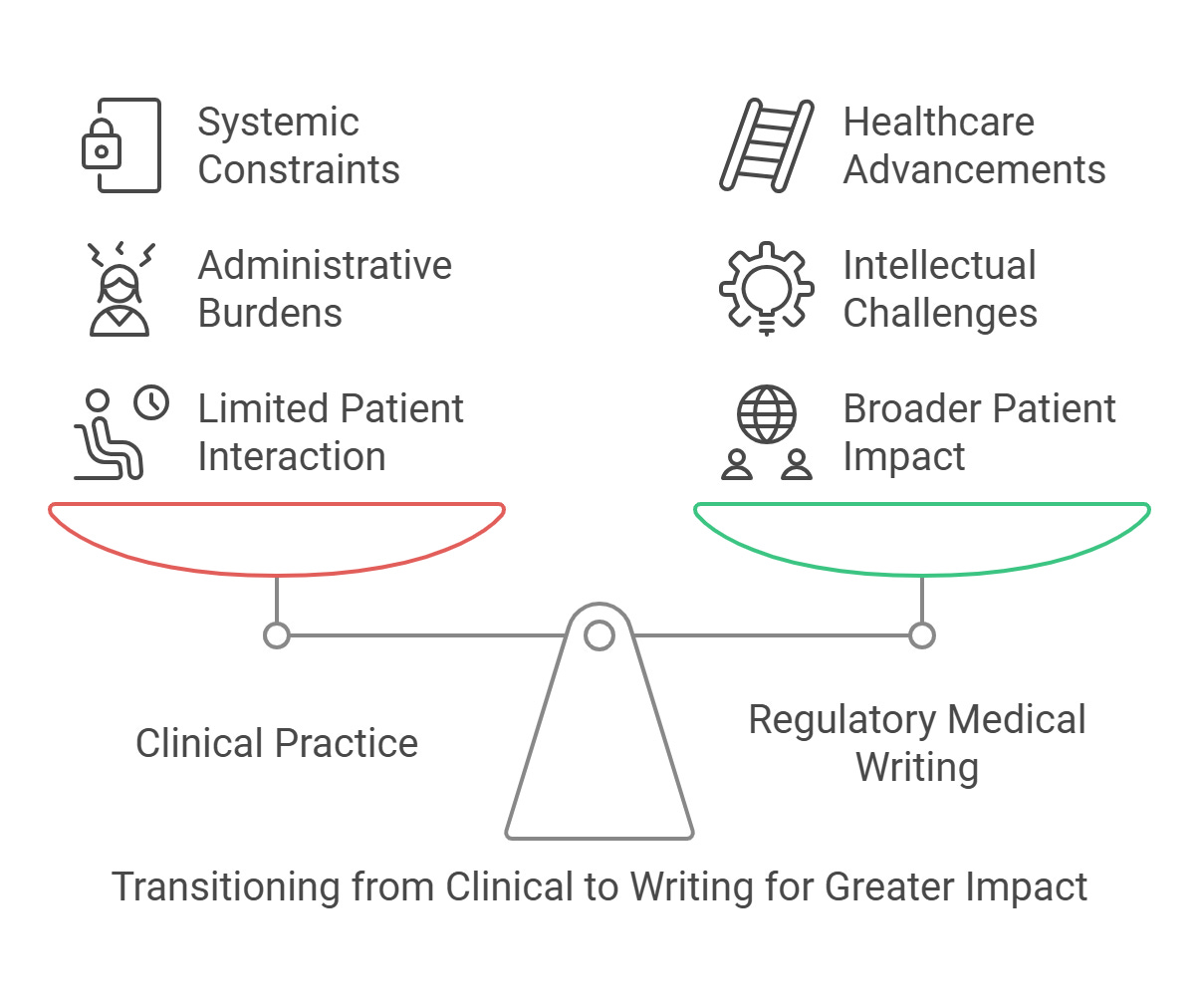
Why Leaving Clinical Practice Can Expand Your Impact
Leaving clinical practice does not mean giving up on helping patients; it opens the door to making a broader and more lasting difference.
Clinical work often comes with frustrations: limited time with patients, inflexible systems, and administrative tasks that overshadow the joy of patient care. These constraints can prevent professionals from focusing on what really matters: the patients. Regulatory medical writing enables you to apply your expertise in a way that supports the development of treatments, offering hope to patients who currently have none.
Your experience communicating medical information or simplifying complex data has already prepared you for this transition.
As a clinician, you are used to summarizing patient outcomes for insurance claims or writing detailed treatment notes. These tasks create clear narratives from complex situations and cases.
This is one of the foundational skills regulatory writers rely on.
How Your Skills Can Save More Lives Through Writing
The skills you developed in clinical practice can be transformed into tools for saving lives on a much larger scale through regulatory medical writing.
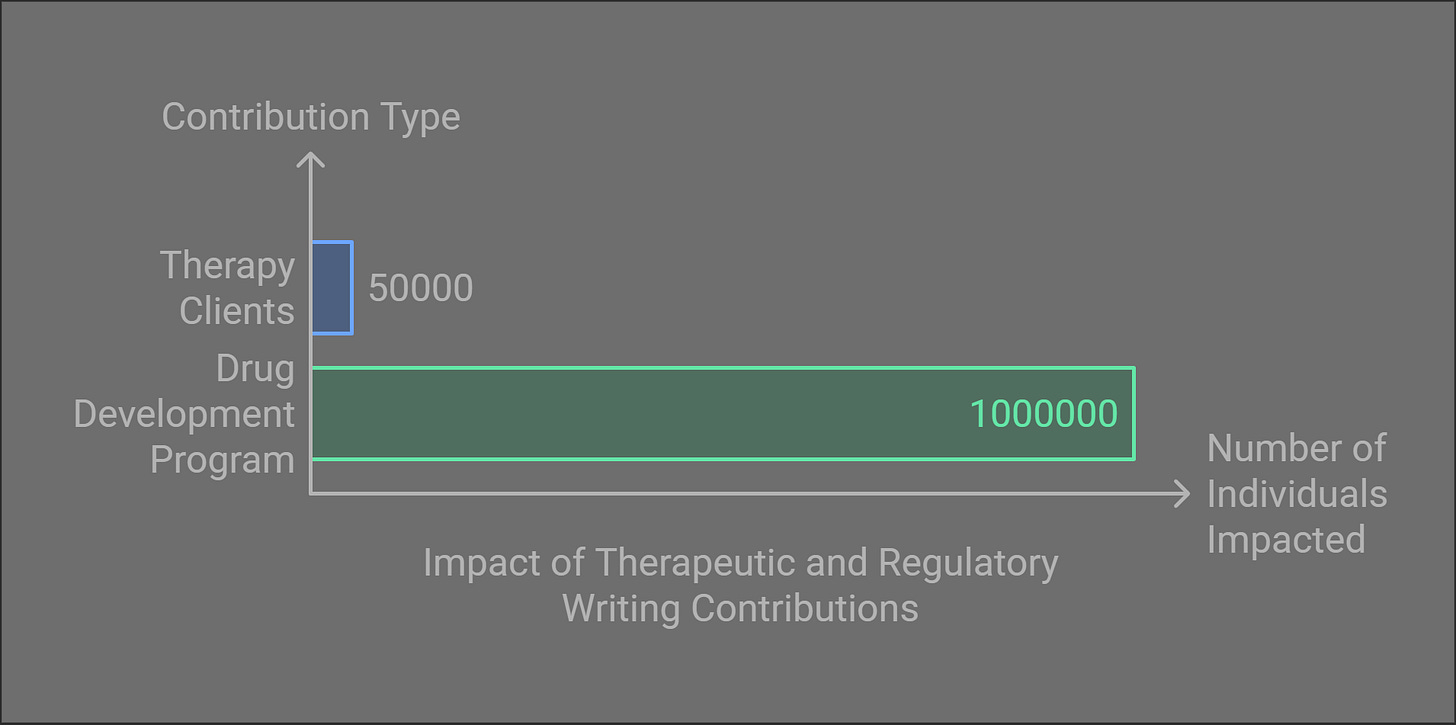
My educational background is as a therapist. You might serve 20-25 clients per week in therapy, potentially impacting 40,000-50,000 individuals over a 40-year career. While this work is undeniably meaningful, the scale of impact in regulatory writing can be staggering.
For example, one drug development program you contribute to may affect millions of patients worldwide, particularly for conditions without effective treatments.
By helping ensure new therapies are safe and effective, your work becomes a catalyst for global change, reaching exponentially more people than direct patient care allows.
Regulatory medical writing allows you to expand your impact beyond what is possible in one-on-one care. You can use your expertise to make a difference for millions of patients worldwide.
Transitioning from Patient Care to Supporting Drug Approvals
Shifting from patient care to supporting drug approvals allows you to leverage your unique strengths to create a lasting impact.
As a clinician, your skills align with the demands of regulatory medical writing:
interpreting data
simplifying complex concepts
collaborating with diverse teams
These abilities are essential for crafting documents regulators rely on to evaluate new therapies. The transition can initially feel intimidating, especially if you're worried about lacking industry experience. Still, the truth is that your clinical background equips you with the very expertise needed to excel in this field.
Consider how often you’ve explained a treatment plan to a patient or communicated findings to other healthcare professionals. These everyday interactions reflect regulatory writers' core competencies:
clarity
precision
collaboration
Many of the skills you take for granted are exactly what the industry values most.
How to Get Started in Regulatory Medical Writing
Launching a career in regulatory medical writing is about building on your existing skills while gaining specialized knowledge of the drug development process and writing techniques.
Success in this field starts with understanding key foundational concepts and tools that regulatory writers rely on daily.
To enter the industry, here’s a concise checklist of the core areas to focus on:
Drug Development Process: Understand the role of each stage of the drug development process.
Core Writing Skills: Simplify complex information and tailor content for regulatory audiences.
Technical Proficiency: Master essential tools like Microsoft Word and document management systems.
Soft Skills Development: Strengthen your soft skills to thrive in cross-functional teams.
Professional Presence: Build a strong LinkedIn profile and network with industry professionals.
Interview Preparation: Translate your experience into industry-relevant skills and prepare for regulatory writing interviews.
Even once you have a good grasp of the above, your first role in the industry is unlikely to be as a regulatory medical writer.
Like most industries, regulatory writing often requires experience “doing the thing” before you can officially land the title. This situation causes a frustrating catch-22 for those trying to break into the industry. Instead, most newcomers start in a different but essential role that lays the perfect foundation for their future writing career.
The role?
Keep reading with a 7-day free trial
Subscribe to The Clinician's Guide to Regulatory Writing to keep reading this post and get 7 days of free access to the full post archives.